New checkpoint inhibitor shows promise in clinical trial for non-Hodgkin’s lymphoma

In their phase-2 study of tisagenlecleucel (marketed as KYMRIAH®), to be published on-line Dec. 1, 2018 in the New England Journal of Medicine, an international team of researchers evaluated 93 patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). They found that 52% of those patients responded favorably to the therapy. Forty percent had a complete response and 12% had a partial response.
Sixty-five percent of those patients—recruited from 27 sites in North America, Europe, Australia and Asia—were relapse free one year later, including 79% of the complete responders. The median progression-free survival for patients in this trial, known as JULIET; NCT02445248, “has not been reached,” the authors note.
This compares favorably with earlier studies based on chemotherapy. The retrospective SCHOLAR-1 study, published in 2017, found that only 7% of patients with refractory DLBCL had a complete response. The median overall survival was a mere 6.2 months. “Outcomes were consistently poor across patient subgroups and study cohorts,” the SCHOLAR-1 authors noted.
More promising data from the JULIET trial “led to the Food and Drug Administration’s approval May 1, 2018 of tisagenlecleucel for treatment of DLBCL,” said study co-author Michael Bishop, MD, Professor of Medicine and Director of the Cellular Therapy Program at the University of Chicago Medicine. “Our current results are a promising sign of the potential for long-term benefit.”
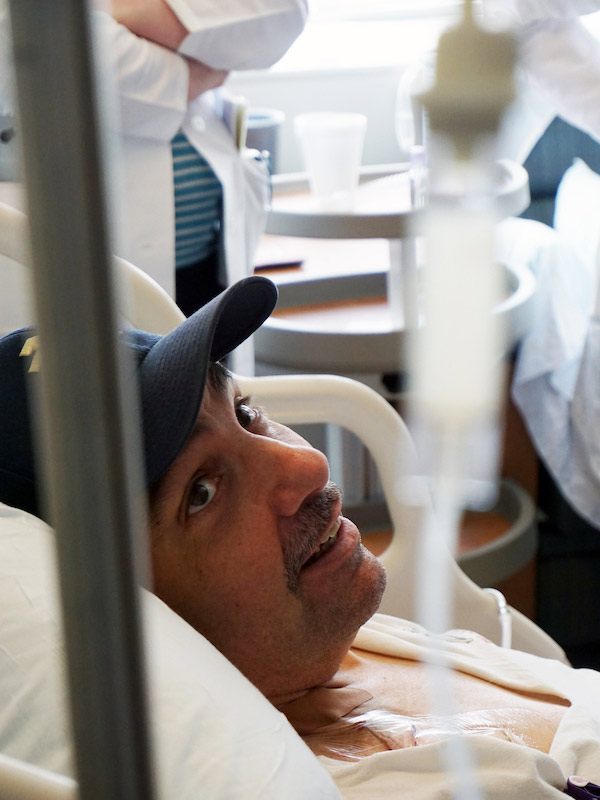
Scott McIntyre, the first patient to receive CAR T-cell therapy for stage 3 DLBCL at the University of Chicago, was pleased to learn about the study results. He is now 30 months post treatment and has had no sign of recurrence. He still gets a transfusion of antibodies every two months, to protect him from the risk of infection that comes with the loss of his normal B cells, but the South Bend, Indiana native quickly returned to his job and seldom misses a Notre Dame football game. He just returned from watching the undefeated team (12 and 0, currently ranked #3 in the country) beat the University of Southern California.
The JULIET trial, based at the University of Pennsylvania, is a single-arm, open-label, global study in adults with relapsed or refractory DLBCL. Patients who were enrolled had received at least two previous lines of therapy.
The treatment process is complicated. The care team takes blood from the patient, extracts T-cells and returns everything else. The T cells were shipped to Novartis’ Morris Plains facility in New Jersey. The team there modified the T lymphocytes, enabling them to find disease-causing B cells that display a surface protein called CD19 and destroy them.
Once the T cells are returned to the hospital and reinfused into the patient, they multiply rapidly, producing millions of offspring. They quickly launch a vigorous assault on cells that display CD19, leading to the rapid destruction of cancerous cells.
How does CAR T-cell therapy work?
The process, a form of immunotherapy, can have severe but generally self-limited side effects. The most common adverse event is cytokine release syndrome (58%), which causes severe flu-like symptoms such as fevers, swelling and low blood pressure. It can also cause neurologic effects such as delirium, but physicians have learned how to control these side effects. No deaths in this trial were attributed to the treatment.
McIntyre experienced mild cytokine release syndrome and some neurologic effects, but responded quickly to drugs designed to limit these side effects.
“The median progression-free survival has not been reached for complete responders,” the authors wrote. The probability of survival at month 12 “was 90% among complete responders.”
“Overall, this is extremely exciting,” Bishop said. “Relapses after 12 months are infrequent. Our first patient, treated in May 2016, has been back at work for two and a half years. This trial demonstrates that CAR T-cell therapy can provide a high rate of durable responses.”
The FDA’s approval of tisagenlecleucel in patients with relapsing or refractory DLBCL is based on the phase-2 JULIET clinical trial, the first multi-center global registration study for Kymriah in adult patients with DLBCL. JULIET is the largest study examining a CAR T-cell therapy in DLBCL. It has enrolled patients from 27 sites in 10 countries across the US, Canada, Australia, Japan and Europe, including: Austria, France, Germany, Italy, Norway and the Netherlands.
The study was sponsored and designed by Novartis Pharmaceuticals Corporation. Medical editorial assistance was provided by ArticulateScience LLC.

Lymphoma care
Tremendous strides have been made in the treatment of lymphoma — a group of blood cancers that begins in the white blood cells that fight infection in the body. UChicago Medicine lymphoma experts are among the first in the nation to offer innovative treatment options, including CAR T-cell therapy.
Learn more about our lymphoma services