Intestinal bacteria from healthy infants prevent food allergy
New research shows that healthy infants have intestinal bacteria that prevent the development of food allergies.
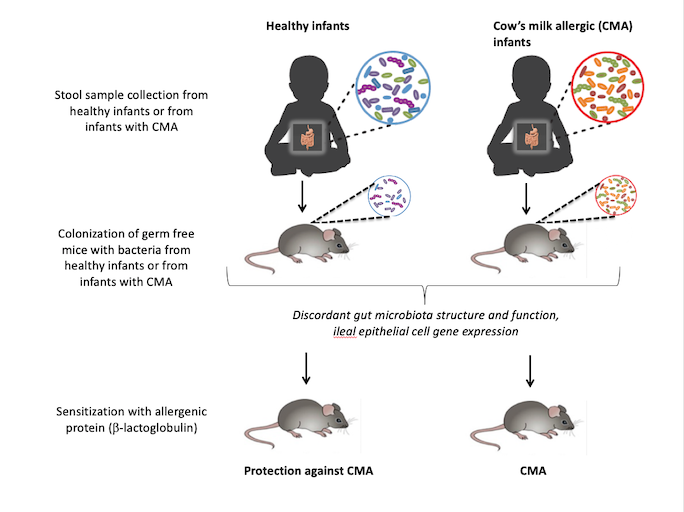
Researchers from the University of Chicago, Argonne National Laboratory and the University of Naples Federico II in Italy found that when gut microbes from healthy human infants were transplanted into germ-free mice, the animals were protected from an allergic reaction when exposed to cow’s milk. Gut microbes from infants allergic to milk did not offer the same protection; mice receiving these bacteria suffered an allergic reaction when given cow’s milk. Cow’s milk allergy is the most common food allergy affecting children.
The study, published this week in Cathryn Nagler, PhD, the Bunning Food Allergy Professor at UChicago and senior author of the study.

The research, funded in part by the National Institute of Allergy and Infectious Diseases, is the result of a long collaboration between Nagler and Roberto Berni Canani, MD, PhD, chief of the Pediatric Allergy Program and CEINGE Advanced Biotechnologies at the University Federico II of Naples, Italy. In 2015,the two worked together on a project that found significant differences in the gut microbiomes of healthy infants and those with cow’s milk allergy. That eventually led them to ask if those differences somehow contributed to the development of the allergy.
The researchers transplanted gut microbes from each of eight infant donors—four healthy and four with cow’s milk allergy—into groups of mice via fecal samples. The mice had been raised in a completely sterile, germ-free environment, meaning they had no bacteria of their own. The mice were fed the same formula as the infants to help the bacteria colonize properly by providing the same sources of nutrients.
Mice that received bacteria from allergic infants suffered from anaphylaxis, a life-threatening allergic reaction, when exposed to cow’s milk for the first time. Germ-free control mice that were not given any bacteria also experienced this severe reaction. Those that received healthy bacteria appeared to be completely protected, however, and did not suffer an allergic reaction.
“These findings demonstrate the critical role of the gut microbiota in the development of food allergy and strongly suggest that modulating bacterial communities is relevant to stopping the food allergy disease burden,” Canani said. “These data are paving the way for innovative interventions for the prevention and treatment of food allergy that are under evaluation at our centers.”
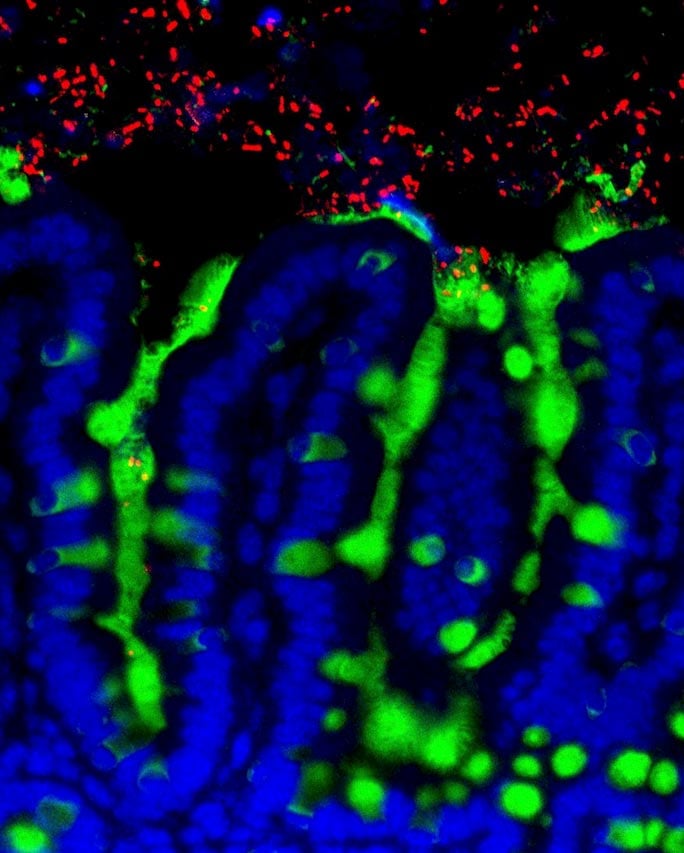
The researchers also studied the composition of microbes in the intestinal tract of the mice and analyzed differences in gene expression between the healthy and allergic groups. This allowed them to pinpoint a particular species, Anaerostipes caccae, that appears to protect against allergic reactions when it is present in the gut.
A.caccaeis part of a class of bacteria, Clostridia, that Nagler and her colleagues identified in recently raised $3.5 million in funding to continue testing these products in animal models before starting human trials.
“It’s exciting because this work is proof of concept for what we’re doing at ClostraBio,” Nagler said. “It shows that we can use metabolic products of the healthy microbiome to develop drugs that protect against food allergy.”
The study, “ If your child has a food allergy or food intolerance, our team will work with you to develop a comprehensive management plan.Pediatric Food Allergies
