Ancient genomes from the Andes highlands reveal novel adaptations

A traditional Aymara ceremony in Copacabana, on the border of Lake Titicaca in Bolivia. (Image from Wikimedia Commons)
A multi-center study of the genetic remains of people who settled thousands of years ago in the Andes Mountains of South America reveals a complex picture of human adaptation from early settlement, to a split about 9,000 years ago between high and lowland populations, to the devastating exposure to European disease in the 16th-century colonial period.
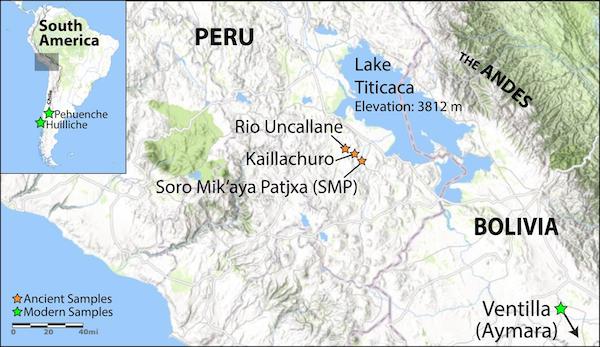
Led by Anna Di Rienzo, PhD, from the University of Chicago; Mark Aldenderfer, PhD, from the University of California, Merced; John Lindo, PhD, JD, from Emory University; and Ricardo Verdugo from the University of Chile, the researchers used newly available samples of DNA from seven whole genomes to study how ancient Andean people—including groups that clustered around Lake Titicaca in Peru and Bolivia, 12,000 feet above sea level—adapted to their environment over the centuries.
In the journal Science Advances, they compared their seven historical genomes to 64 modern-day genomes from a current highland Andean population, the “agropastoral” Aymara of Bolivia, and the lowland hunter-gatherer Huilliche-Pehuenche in coastal Chile.
The goal was to identify genetic adaptations that took place from (1) the early migration into the Andean highlands, to the (2) split between low- and high-altitude populations about 8,750 years ago, through the (3) arrival of Spanish explorers in the 1530s and their near annihilation of many lowland communities of South America.
“We have very ancient samples from the high Andes,” said Di Rienzo. “Those early settlers have the closest affinity to the people who now live in that area. This is a harsh, cold, resource-poor environment, with low oxygen levels, but people there adapted to the climate and the agrarian lifestyle.”
Adjustments to diet and altitude
The study, “The Genetic prehistory of the Andean highlands 7,000 years BP through European contact,” uncovered several unexpected features. The strongest genetic signal the researchers found was a gene called MGAM (maltase-glucoamylase) an intestinal enzyme. It plays an important role in the digestion of starchy foods such as potatoes. This may represent “an adaptive response to greater reliance upon starchy domesticates,” the authors wrote.
“Positive selection on the MGAM starch digestion enzyme is very interesting, said study author John Lindo, PhD, from Emory. “The potato was first domesticated in the Andes and there is some evidence that MGAM may serve as an alternative starch digestion pathway under stressful conditions.”
The early presence of this gene in Andean peoples suggests “a significant shift in diet from one that was likely more meat based to one more plant based,” said Aldenderfer, an anthropologist. “The timing of the appearance of the gene is quite consistent with what we know of the paleo-ethno-botanical record in the highlands.”
Although Andean settlers consumed a high-starch diet after they started to farm, their genomes did not develop additional copies of the starch related amylase gene, commonly seen in European farming populations.
Even though the highlanders lived in altitudes above 8,000 feet, which meant reduced oxygen, frequent frigid temperatures and intense ultra-violet radiation, they never developed the responses to hypoxia seen in natives of other high-altitude settings, such as Tibet.
The Andeans may have adapted to high altitude hypoxia “in a different way, via cardiovascular modifications,” the researchers suggest. They found evidence of alterations in a gene called DST, which is associated with the formation of cardiac muscle. Andean highlanders tend to have enlarged right ventricles. This may have improved oxygen intake, enhancing blood flow to the lungs. But this enlargement is known, in other settings, to increase the risk of pulmonary hypertension.
Protection from the environment
The researchers also found that highland Andeans experienced much smaller than expected population declines following contact with European explorers who first came to South America in the 1530s.
The harsh mountain environment may have discouraged European invaders.
This appears to have protected highland dwellers from frequent exposure to lethal foreign diseases, such as smallpox. In the lowlands, more than 90 percent of residents died from diseases they had never experienced. But the people living in the upper Andes had only a 27 percent population reduction.
The ancient genomes also revealed early selection for immune-related genes soon after the arrival of Europeans, suggesting that Andeans who survived slowly gained the ability to respond to the newly introduced European pathogens.
“Contact with Europeans had a devastating impact on South American populations, such as the introduction of disease, war, and social disruption,” explained Lindo. “By focusing on the period before that, we were able to distinguish environmental adaptations from adaptations that stemmed from historical events.
“In our paper,” said Alendorfer, “there was none of this prioritization of genes at the expense of archaeological data. We worked back and forth, genetics and archeology, to create a narrative consistent with all of the data at hand.”
The National Institutes of Health, the National Science Foundation, the American Philosophical Society, the National Geographic Society, the H. John Heinz III Charitable Trust, the University of Chicago Provost’s Postdoctoral Scholarship and the Chilean National Council of Science and Technology funded this study. Additional authors were David Witonsky and John Novembre, University of Chicago; Randall Haas, UC Davis; Courtney Hofman, University of Oklahoma; Mario Apata and Mauricio Moraga, University of Chile; James Watson, University of Arizona; Carlos Llave, Peruvian Register of Professional Archaeologists; Cynthia Beall, Case Western University; and Christina Warinner, Max Planck Institute, Germany.

Anna Di Rienzo, PhD
Anna Di Rienzo, PhD, is Professor in the Department of Human Genetics. Her lab works to describe the amount and patterns of genetic variation in human populations, and to explain the forces that shape and maintain this variation.
Learn more about Dr. Di Rienzo